Confidential Automatic Monitoring, Examination, and Recognition of Disease Activity (CAMERA) study
Patients with neurodegenerative disorders such as Parkinson’s disease (PD) often make long journeys to visit their physicians and trial multiple therapies and medications to manage their symptoms. Physicians use clinical assessments to inform drug regimens for their patients, however these assessments are performed infrequently, and symptoms may change vastly in between visits. This research aims to create a video-based data collection platform specifically designed to assess PD symptoms by capturing detailed facial expressions, hand movements, finger tapping, and eye movements. Using advanced machine learning algorithms, the collected data is analyzed to generate comprehensive, data-driven reports for clinicians and researchers. This will enhance the understanding of PD symptoms and offer an efficient, scalable method for assessing disease severity through non-invasive video-based assessments.
Principal Investigator: Dr. Martin McKeown
Primary contact: Michael Grundy
604-822-9722
michael.grundy@ubc.ca
Somatotopy in Parkinson’s disease
"Somatotopy" refers to how areas of the brain are organized according to the body part they affect. The striatum is the brain region that coordinates complex thinking and movement. Plasticity refers to changes in connections within the brain, which can happen to make up for changes that are related to PD. This study aims to assess these changes in connections within the brain in Parkinson's disease (PD) using PET (Positron Emission Tomography) and fMRI (functional Magnetic Resonance Imaging) on the Hybrid PET/MRI scanner. PET and fMRI imaging together allow investigate changes in the striatum in people living with Parkinson's disease when compared to people without Parkinson’s disease.
Principal Investigator: Dr. A. Jon Stoessl
Primary contact: Sahib Dhaliwal
604-827-1353
sahib.dhaliwal@ubc.ca
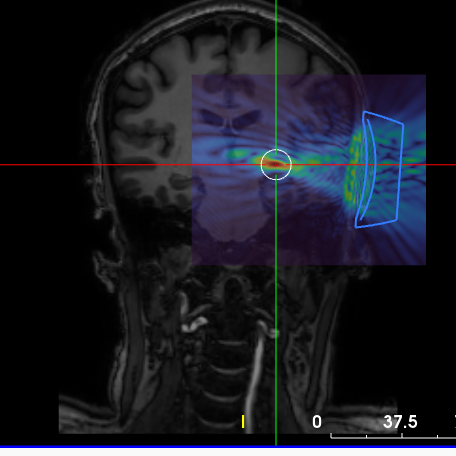
Low Intensity Focused Ultrasound for Parkinson’s Disease Tremor
In this study, we aim to compare the effects of targeting two different brain regions, the traditionally treated VIM and the zona incerta (ZI), using LIFUS for tremor control in Parkinson’s disease. Past research has shown that the ZI may be an important area for treating tremor and other Parkinson’s symptoms like stiffness and uncontrolled movements. Our goal is to understand how these brain regions contribute to Parkinson’s tremor and how the network responds to LIFUS. The knowledge gained will contribute to developing more effective treatments for Parkinson’s disease in the future.
Principal Investigator: Dr. Martin McKeown
Primary contact: Maggie Vuong
604-822-0345
maggie.vuong@ubc.ca
Individualized prediction of medication wearing off using wearables
Parkinson’s disease is most commonly treated with levodopa, and while this treatment method has shown to significantly help reduce symptoms, many patients also experience “wearing-off” (WO) episodes, where symptoms re-emerge prior to their next dose. This study aims to investigate whether these episodes can be predicted – and ultimately prevented – using machine learning to assess biomarkers from the autonomic nervous system such as electrodermal activity (EDA), a measure of sweating from the skin. To monitor EDA and WO episodes, participants will be asked to wear a wrist sensor for 72 hours and complete questionnaires on an hourly basis via their mobile device.
Principal Investigator: Dr. Martin McKeown
Primary contact: Juana Ayala Castaneda
mckeown.lab@ubc.ca
Recent Publications
Anxiety is associated with increased risk of suicidality in Parkinson’s disease. Lam JS, Tosefsky KN, Zhu J, et al. (2026). Journal of Parkinson’s Disease. 2026;0(0). doi:10.1177/1877718X251410887
A randomized safety and feasibility crossover trial of two Mediterranean-ketogenic interventions in individuals with Parkinson’s disease. Tosefsky K, Lam JS, Wang YN, et al. (2026). Journal of Parkinson’s Disease. 2026;0(0). doi:10.1177/1877718X261418986
Individualising galvanic vestibular stimulation further improves visuomotor performance in Parkinson’s disease. Menon, A., Vigneswaran, M., Zhang, T., Sreenivasan, V., Kim, C., & McKeown, M. J. (2025). Bioengineering, 12(5), 523. https://doi.org/10.3390/bioengineering12050523
EEG dynamical features during variable-intensity cycling exercise in Parkinson’s disease. Alizadeh, Z., Arasteh, E., Mirian, M. S., Sacheli, M. A., Murray, D., Appel-Cresswell, S., & McKeown, M. J. (2025). Frontiers in Human Neuroscience, 19, 1571106. https://doi.org/10.3389/fnhum.2025.1571106
Disease-Modifying Trials in Treated Parkinson’s Disease: “Stable Treated” Does Not Equate with Biological Stability. Mouradian, M. M., Stoessl, A. J., & Lang, A. E. (2025). Movement Disorders, 40(9), 1778–1790. https://doi.org/10.1002/mds.30259. PMC 12485585
Sex and gender differences in the molecular etiology of Parkinson’s disease: considerations for study design and data analysis. Schaffner, S. L., Tosefsky, K. N., Inskter, A. M., Appel-Cresswell, S., & Schulze-Hentrich, J. M. (2025). Biology of Sex Differences, 16(1), 7. https://doi.org/10.1186/s13293-025-00692-w
A generalized framework for in vivo detection of dopamine release using positron emission tomography. Hanania, J. U., Bevington, C. W. J., Cheng, J. K., et al. (2025). Journal of Cerebral Blood Flow & Metabolism. Published online 19 Sept (2025). https://doi.org/10.1177/0271678X251362958
Feature Space-Guided Denoising of Noisy 4D Data: Applications to Dynamic PET Imaging and Dual-Calibrated Functional MRI. Bevington, C. W. J., et al. (2025). IEEE Transactions on Radiation and Plasma Medical Sciences. https://doi.org/10.1109/TRPMS.2025.3608506